
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Evaluations of phylogenetic proximity in a group of 67 dogs with
osteosarcoma: a pilot study
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Canine Periosteal Osteosarcoma
Images added / Abbildungen hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
New subcategory added / Neue Unterkategorie hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
Images added / Abbildungen hinzugefügt 01.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Heart valvular diseases



Abdominal and pelvic ultrasonography in healthy golden retriever dogs, carriers and affected by gradual muscular dystrophy

Angelica Paula Grando I; Arani Nanci Bonfim Mariana II; Maria Angelica Miglino II; Franklin Almeida Sterman II and IV; Mayana Zatz III; Luciane Maria Kanayama IV; Matheus Levi Feitosa Tjara II; Daniele dos Santos Martins II; Adriana Caroprezo Morini II; Juliana Passos Alves dos Santos II; Leandro Fadel II; Flavio Ribeiro Alves II; Carlos Eduardo Ambrósio II, 1

RESUME
The Duchenne muscular dystrophy (DMD) is a type of muscular dystrophy in humans characterized by a genetic disorder linked to chromosome X. The Golden Retriever dog carrier muscular dystrophy (GRMD) has been intensively studied and considered the most representative model for the disease observed in humans. Thus, in order to verify abnormalities in internal organs in these animals, we performed the ultrasound examination of 24 golden retriever dogs healthy and affected by muscular dystrophy. The ultrasound examination of the liver increased GRMD diagnosed moderate to severe, including hepatic vessels and their branches and increased echogenicity of the gallbladder and urinary bladder. However, there were no clear images of changes in the spleen and in the vessels branches of the aorta. From this, we believe that ultrasound is in a useful procedure to the assessment of abdominal organs in dogs affected by muscular dystrophy.
ABSTRACT
Duchenne muscular dystrophy (DMD) is one type of human muscular dystrophy's Characterized by a genetic disorder linked to the X chromosome. The Golden Retriever muscular dystrophic (GRMD) Has Been Studied extensively and Considered the best resembling model to the human disease. Therefore, for Identifying internal organs abnormality in GRMD, abdominal and pelvic ultrasonography was Performed in 24 golden retriever dogs, either healthy or muscular dystrophic in different levels of disease. The GRMD ultrasonographic exams diagnosed moderate to severe liver enlargement, including hepatic vessels and Their branches and Increase of echogenicity in gallbladder and urinary bladder. However was not-Clearly Recognized pathologic images from spleen and aortic vessels Were accessed. Therefore, we believe, the ultrasonographic exam was an useful procedure to the assessment of abdominal organs in dogs affected by muscular dystrophy.
INTRODUCTION
The progressive muscular dystrophies (MWD's) are a group of human diseases characterized by progressive and irreversible degeneration of skeletal muscle (Zatz & STEPS Bueno, 1995). The Duchenne muscular dystrophy (DMD), a genetic recessive inheritance, has an incidence of 1 per 3,000 births male, becoming symptomatic early in life (three to five years old) and progressing to failure ambulate during the period near the end of the first decade (Engel, 1990;. NICHOLS et al, 1994; Zatz & STEPS Bueno, 1995).
Brazeau et al. (1992) and MORINI et al. (2008) highlight the importance of studying human diseases in animal models for preclinical evaluation of possible treatment modalities and to detect abnormalities that may alter the pharmacokinetics of administered therapeutics. In the case of the DMD, the most studied animal model is the murine (X-linked murine dystrophy - MDX), however, a canine model (canine X-linked muscular dystrophy - cxmd) due to its size and the symptomatology similar to DMD seen in humans, has been shown above (NICHOLS et al., 1994; Bergman et al., 2002). Although registered in various breeds of dogs, such dystrophy has been best characterized in the Golden Retriever (Golden Retriever Muscular Dystrophy - GRMD) (Bergman et al., 2002).
Several other muscular organ systems and non-muscle can also be changed in humans and dystrophic animals. Studies Nowak et al. (1982); BAROHN (1988); Miyatake et al. (1989); Moriuchi et al. (1991); Berry et al. (1992); Brazeau et al. (1992) and Stein et al. (2002) spoke about voltage increases observed in the hepatic portal vein, as well as sagging parade gallbladder caused by the absence of dystrophin gene in mdx mice. Furthermore, those authors also reported malabsorption syndrome and diarrhea processes assigned to pancreatic dysfunction in humans.
Thus, this work aimed to verify the normal sonographic appearance and abnormal abdominal and pelvic organs in golden retriever dogs in varying degrees of development of muscular dystrophy.
MATERIAL AND METHODS
Animals
The survey was conducted after the approval of the Bioethics Committee of the Faculty of Veterinary Medicine and Animal Science of the University of São Paulo (USP-FMVZ). 24 dogs golden retriever between males and females were used, puppies and adults, and affected by progressive muscular dystrophy. The animals came from the experimental kennel Golden Retriever Muscular Dystrophy (GRMD - Brazil), Department of Surgery (Figure 1a.). Of the 24 animals evaluated, 15 were puppies six months and nine adults were between two and five years old. Among the young, four were healthy (two females and two males), five patients (females) and six affected (males). Among adults, four were healthy (two males and two females), four carriers (females) and one affected (male) (Fig. 1B). The animals were kept under veterinary medical care, food, hygiene and permanent recreation.

Genetic diagnosis
Genotypic identification of muscular dystrophy was conducted by the Human Genome Studies Center at the University of São Paulo (IB-USP), shortly after the birth from umbilical cord blood, by genomic DNA extraction, using the kit GFX Genomic Blood DNA Purification Kit (GE Healthcare). Confirmation of muscular dystrophy was also performed by high levels of serum creatine kinase enzyme, Kerkis et al. (2008).
Ultrasound examination
We proceeded to the ultrasonographic examination through abdominal and pelvic scan for visualization and characterization of their structures. For this, we used a portable ultrasound device Tokimec ®, CS-3030 model with a 7.5 MHz linear transducer and convex 5,0MHz. Images were recorded on video copy processor printer P66E model, Mitsubishi ®. As a contact means for transmitting ultrasound was used Carbogel ®.
For the ultrasound examination, the dogs were submitted to solid fasting for 12 hours and water for at least 1 hour before the exam. After shaving the abdominal and pelvic region, followed by the exam, set at random, without exams executor of knowledge, of which animal was normal or affected, making a blind study group. The abdominal and pelvic organs were analyzed for its topography and sonographic appearance.
RESULTS AND DISCUSSION
Studies Nowak et al. (1982), BAROHN et al. (1988), Miyatake et al. (1989), Moriuchi et al. (1991), Berry et al. (1992), Brazeau et al. (1992) and Stein et al. (2002) demonstrated changes in parenchymatous internal organs and muscle, both in patients with Duchenne and animals with muscular dystrophy. Similar results obtained in this experiment are described in Table 1. Similarly, in this study were observed striking changes in echo texture and size standards, dimensions and volume of the abdominal and pelvic structures. Recently, BRUMITT et al. (2006) described for dogs of the same breed just a hepatomegaly linked to the abdominal serous slight modifications, as opposed to detailed modifications of the abdominal structures shown and emphasized (Table 1).

Although known hepatomegaly neonatal described by ROTHUIZEN (2001), this change was observed in all dystrophic dogs of the group studied, corroborating the information described in humans with Duchenne (Stein et al., 2002) in hypertrophic muscular dystrophy in cats (BERRY et al., 1992) and mdx mice (Brazeau et al., 1992). In addition, the hepatomegaly was also observed in the other six puppies (both healthy and patients) and three adults. In this way, we confirm these findings in GRMD adult dogs, like described BRUMITT et al. (2006). They added further microscopic findings described by Santos et al. (2007) who described the loss of trabecular organization and cordonal of hepatocytes, the presence of mononuclear cells in the sinusoids and vessels, hyperplasia of the space holder and the obvious fibrous tissue, and areas of congestion and ischemia in dogs affected by muscular dystrophy muscle.
The observed decrease in liver echogenicity by Berry et al. (1992) in dystrophic cats does not agree with the findings of liver hyperechogenicity in young dogs and dystrophic this research, when we observe that nine puppies between healthy and carriers, and six adults also showed liver hyperechogenicity. Though often such sonographic findings constitute frames of fatty infiltration, liver disease by steroids, diabetes, lymphoma, and toxic liver disease (Mamprim, 2004), we attribute the change to excess metabolites derived from degraded compounds of skeletal muscle and the fact that these animals high ALT present, with a response to the effect of muscular dystrophy (MORINI, 2008).
The high caliber of the branches of the hepatic vein constitutes an abnormal finding, when compared to values reported in the literature and described by Salgado et al. (2007). Based on the observations made by Chetboul et al. (2004) and Pellegrino et al. (2007) when they studied, respectively, dystrophic dogs and healthy, we believe in a concomitant heart disease as a possible cause for the change observed ultrasonographically, still confirming the observations made by Mamprim (2004) in a similar study. Similarly, samiel (2000) reports the advanced age as a developer agent in the development of cardiomyopathies and hepatic venous congestion of the system dystrophic dogs.
The presence of bile sediment remains to be established, although it is common to observe it in aged animals, obese, sedentary or endocrinopatas (CENTER, 1992; Mamprim, 2004). The evaluated dogs had severe dysplasia due to ingestion of small quantities of food, it is thus justifying the occurrence of biliary stasis and consequent formation of dense bile and the gallstones in four of these animals (Figure 2). Such gallstones can also be associated with changes in cholesterol levels and ionic components of bile as described by Mamprim (2004). The contraction of smooth muscle deficiencies gallbladder observed in this study are supported in remarks NOWAK et al. (1982), BAROHN et al. (1988) and Miyatake et al. (1989) suggested when the systemic dysfunction of mdx mice vascular smooth muscle and gastric motility in humans with Duchenne.
Although splenomegaly were present in dystrophic individuals studied by STEIN et al. (2002) and Berry et al. (1992), in this study, there was no increase in spleen size in affected dogs. In their studies, TANNOUZ (2004) highlighted the spleen volume decreased in cases of cachexia and dehydration, conditions observed frequently in dystrophic dogs. These findings are also seen in the work of Moriuchi et al. (1991) found that loss of body weight in human patients affected by DMD due to reduced skeletal muscle. Additionally, dogs were studied dystrophic smaller in size and body weight compared to healthy dogs of the same breed and age, with this feature becoming more evident with disease progression.
In ultrasonographic evaluation three dystrophic dogs showed hyperechoic dots suspended in the content of the urinary bladder. Studies by MACLEO et al. (2003) and TUBBS & OAKES (2004) in human DMD also found lower urinary tract disorders such as, for example, short retention capacity dyssynergia and hyperreflexia of the sphincter muscle.
Although Muscatelli et al. (1994) and pillers et al. (1990) to report the possibility of congenital adrenal hypoplasia in patients with Duchenne muscular dystrophy and BERRY et al. (1992), and Carpenter et al. (1989) have observed the presence of mineralization of the adrenal glands in two dystrophic cats in this study, such a change was detected only in isolation (right adrenal gland measuring 4.1 x 2,1cm) on an affected adult dog.
The pancreas was visualized in five of 24 dogs studied GRMD, identifying the pancreaticoduodenal vein, and pancreatic duct (Figure 3A and B). The body pattern of low weight and low fat in animal studies may have favored the identification of the pancreas, corroborating Berford assessments (2004), who found that for lean animals, the pancreatic margins are more discretely defined. In an affected puppy was also observed echogenicity high pancreatic, however, consisting of only an occasional finding.
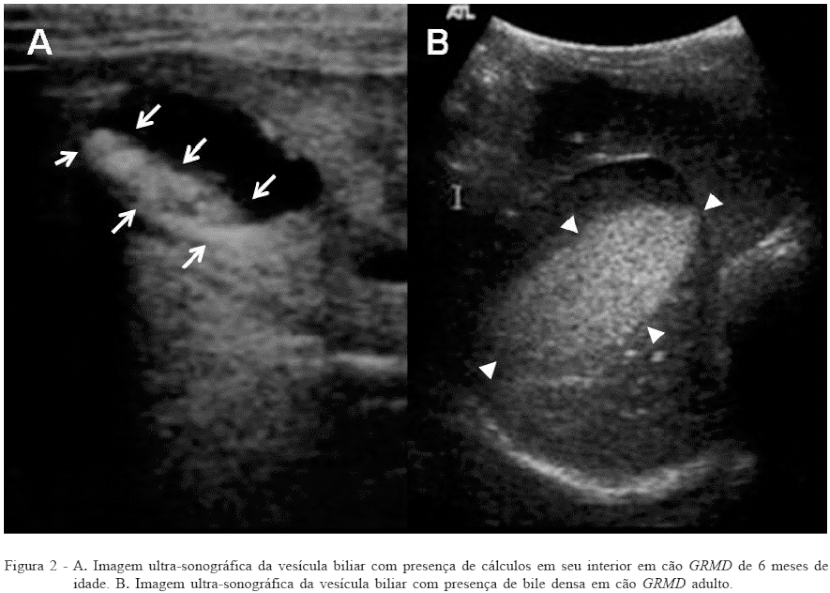
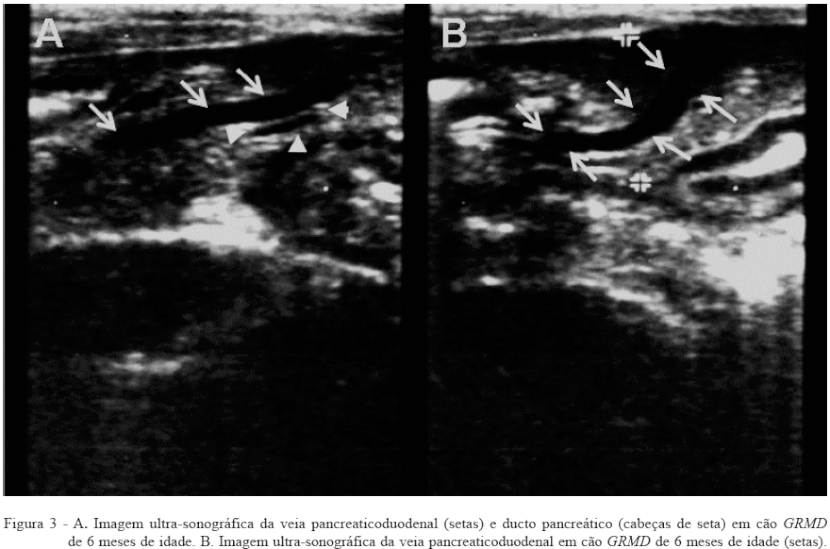
CONCLUSIONS
The ultrasound examination consisted in a non-invasive, rapid technique, easily accessible and sensitive in identifying parenchymal echogenicity changes of abdominal and pelvic organs in dogs affected by muscular dystrophy. Increased echogenicity and organ architecture pattern changes as the liver and pancreas, observed through ultrasound, are effective signals to make predictions about the evolution of muscular dystrophy in dogs, thus becoming a model suitable animal to study similar changes in humans.
REFERENCES
BAROHN, RJ et al. Gastric hypomotility in Duchenne's muscular dystrophy. New England Journal of Medicine, v.319, p.15-18, 1988. [Links]
Berford, RM Pancreas. In: CARVALHO, CF (Ed.) Ultrasound in small animals.. Sao Paulo: Roca, 2004. p.75-83. [Links]
BERGMAN, RL et al. Dystrophin-deficient muscular dystrophy in the Labrador Retriever. Journal of the American Animal Hospital Associassion, v.38, n.3, p.255-261, 2002. [Links]
BERRY, CR et al. Radiographic and ultrasonographic features of hypertrophic feline muscular dystrophy in two cats. Veterinary Radiology and Ultrasound, v.33, n.6, p.357-364,1992. [Links]
Brazeau, GA et al. Serum and organ indices of the mdx dystrophic mouse. Research Communications in Chemical Pathology and Pharmacology, V.77, n.2, p.179-189, 1992. [Links]
BRUMITT, JW et al. Radiografic features of golden retriever muscular dystrophy. Veterinary Radiology & Ultrasound, V.47, n.6, p.574-580, 2006. [Links]
CARPENTER, JL et al. Muscular dystrophy feline with dystrophin deficiency. American Journal Pathology, v.135, p.909-919, 1989. [Links]
CENTER, SA Pathophysiology and laboratory diagnosis of liver diseases. In: ETTINGER SJ (Ed.) Treaty of Veterinary Internal Medicine:. Dog and cat diseases. Sao Paulo: Manole, 1992. p.1487-1546. [Links]
CHETBOUL, V. et al. Tissue Doppler imaging detects early asymptomatic myocardial abnormalities in the dog model of Duchenne's cardiomyopathy. Preclinical Research, v.25, p.1934-1939, 2004b. [Links]
ENGEL AG muscle diseases (myopathies) and neuromuscular junction. In: Wyngaarden JB; SMITH Jr LH (Ed.) Cecil. - Internal Medicine treaty. Rio de Janeiro: Guanabara Koogan, 1990. p.1988-2001. [Links]
Kerkis, I. et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: Local or systemic Journal of Translational Medicine, v.6, p.35, 2008. [? Links]
Mamprim, MJ liver and gallbladder. In: OAK CF (Ed.) Ultrasound in small animals.. Sao Paulo: Roca, 2004. p.51-73. [Links]
Miyatake, M. et al. Possible systemic smooth muscle layer dysfunction due to a deficiency of dystrophin in Duchenne muscular dystrophy. Journal of the Neurological Sciences, v. 93, p.11-17, 1989. [Links]
MORINI et al. Analysis of strengths and serum concentrations of cyclosporine A in Breed Dog Golden Retriever normal or affected by muscular dystrophy. Brazilian Journal of Veterinary Research and Animal Science. v.45, n.2. p.131-137, 2008. [Links]
Moriuchi, T. et al. Autopsy study on the heart, liver, kidney and brain in distrophy muscular dystrophy. Tokushima Journal of Experimental Medicine, v.38, p.5-13, 1991. [Links]
Muscatelli, F. et al. Mutations in the DAX-1 gene give rise to BOTH adrenal hypoplasia congenital X-linked and hypogonadotropic hypogonadism. Nature, v.372, n.6507, p.672-676, 1994. [Links]
NICHOLS, PL et al. Gene therapy in Duchenne muscular dystrophy. Nih Guide, v.23, n.7, 1994. Access on Jun 11 2005. Online. Available at: <http://grants2.nih.gov/grants/guide/pa-files/PA-94-040.html> [Links] .
NOWAK, TV et al. Gastrointestinal manifestations of the muscle dystrophies. Gastroenterology, V.82, p.800-810, 1982 [Links]
PELLEGRINO, A. et al. Echocardiographic parameters for standardization of Golden Retriever dogs clinically healthy. Rural Science, v.37, n.4, p.1039-1044, 2007. [Links]
Pillers, DA et al. Aland Island eye disease (Forsius-ocular albinism Eriksson) and an Xp21 deletion in a patient with Duchenne muscular dystrophy, glycerol kinase deficiency, and congenital adrenal hypoplasia. American Journal of Medical Genetics, v.36, n.1, p.23- 28. 1990. [Links]
ROTHUIZEN, J. Liver Diseases and diseases of the biliary tract. In: DUNN JK (Ed.) Treaty of medicine for small animals.. Sao Paulo: Roca, 2001. p.444-493. [Links]
SALGADO, SAB et al. Identification of regions corresponding to the hepatic lobes of dogs by means of ultrasound. Brazilian Animal Science, v.8, n.3, p.545-558, 2007. [Links]
Samiel, HV Genetic surgery for muscular dystrophy in golden retrievers. Genome News Network, June, 2000. Access in 2003. Online. Available in: http://www.genomenewsnetwork.org/articles/06_00/muscular_dystrophy.shtml. [Links]
SANTOS, FM et al. Microscopic study of the liver and pancreas of Golden Retriever dogs affected by muscular dystrophy. International Journal of Morphology, v.25, n.1, p.192-93, 2007. [Links]
STEIN, MT et al. Fatigue, decrease interest in play, motor delay, and elevated liver function tests in the 4-year-old boy. Journal of Developmental and Behavioral Pediatrics, v.23, n.1, p.37-41, 2002. [Links]
TANNOUZ, VGS Spleen. In: OAK CF (Ed.) Ultrasound in small animals.. Sao Paulo: Roca, 2004. p.85-99. [Links]
TUBBS, R. S & OAKES WJ Urinary incontinence in a patient with Duchenne muscular dystrophy and cord in the regular position with fatty filum terminale. Child's Nervous System, v.20, p.717-719, 2004. [Links]
Zatz, M .; STEPS BUENO, MR hereditary myopathies: advances of the last two years. In: NITRINI R. et al Pipelines in neurology.. Sao Paulo: Clinical Neurological HC-FM, USP, 1995. p.71-78. [Links]
Submitted 06:05:08
Approved at 04:07:08
Share this article / Teilen Sie diesen Artikel
Diese Webseite wurde mit Jimdo erstellt! Jetzt kostenlos registrieren auf https://de.jimdo.com








