
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Evaluations of phylogenetic proximity in a group of 67 dogs with
osteosarcoma: a pilot study
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Canine Periosteal Osteosarcoma
Images added / Abbildungen hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
New subcategory added / Neue Unterkategorie hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
Images added / Abbildungen hinzugefügt 01.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Heart valvular diseases



Imaging and surgical outcomes of spinal tumors in 18 dogs and one cat

Besalti, Omer et al. “Imaging and Surgical Outcomes of Spinal Tumors in 18 Dogs and One Cat.” Journal of Veterinary Science 17.2 (2016): 225–234. PMC. Web. 11 Mar. 2017.

Abstract
Clinical and magnetic resonance imaging (MRI) findings, histological appearances and surgical outcomes of 18 dogs and one cat with spinal tumors are presented. Medical records of the cases admitted for spinal disorders were reviewed, and cases of spinal tumors that were diagnosed by MRI and confirmed by histological examination were included in this study. T1 weighted, T2 weighted and contrast enhanced T1 weighted images were taken and interpreted to evaluate the spinal tumors. The tumors were diagnosed as: meningioma (n = 6), ependymoma (n = 1), nerve sheath tumor (n = 4), metastatic spinal tumor (n = 3), osteosarcoma (n = 2), osteoma (n = 1), rhabdomyosarcoma (n = 1), and nephroblastoma (n = 1). Thirteen cases underwent surgical operation and the remaining six cases were euthanized at the request of the owners. The neurological status of the surgical cases did not deteriorate, except for one dog that showed ependymoma in the early period after the operation. These results indicate the potential for surgical gross total tumor removal of vertebral tumors to provide better quality of life and surgical collection of histological specimens for definitive diagnosis. For effective case management, dedicated MRI examination is important to accurate evaluation of the spinal tumors, and surgical treatment is useful for extradural and intradural-extramedullary spinal tumors.
Introduction
Spinal neoplasia involves the spinal cord, dura, exiting peripheral nerves, or paraspinal tissues (e.g., the vertebrae and ligaments) and results in clinical signs of spinal cord dysfunction [2]. Spinal tumors can be classified as primary, originating from spinal meningeal and paraspinal tissues, or as secondary, metastazing from other locations. They are also classified by anatomic location, in relation to the dura and spinal cord, and based on whether there is spinal involvement [7,13].
Magnetic resonance imaging (MRI) has a high soft-tissue resolution and plays a central role in depiction of spinal tumors, allowing them to be classified as extradural, intradural-extramedullary or intramedullary, which is very useful in tumor characterization. Extradural tumors are the most common spinal tumors in dogs and cats. These tumors include primary and secondary mesenchymal tumors (osteosarcoma, fibrosarcoma, and chondrosarcoma), hemangiosarcoma, multiple myeloma and other plasma cell tumors, liposarcoma, and lymphosarcoma [2,8,20,26]. Intradural but extramedullary tumors include meningiomas and nerve sheath tumors [6,32,33]. Meningiomas are most often found in the cervical area, followed by the lumbar area. Intramedullary tumors, which include astrocytomas, oligodendrogliomas, ependymomas and nephroblastomas, arise from cells within the spinal cord parenchyma [9,13,16,33]. Additionally, there is an unusual intradural but extramedullary tumor that has been referred to by various terms, including neuroepithelioma and spinal cord blastoma [4,13,29].
Treatment options for spinal tumors include surgical removal, radiation therapy and chemotherapy [10,11,12,17]. The prognosis depends on the degree of local resection, degree of spinal infiltration, spinal cord damage before and during surgery, surgeon's experience with spinal neoplastic conditions, and tumor type [2]. This study was conducted to describe the clinical and MRI findings and histological features of 19 cases of spinal tumor, and to evaluate the surgical outcomes of 12 dogs and one cat.
Materials and Methods
Medical records at the Ankara University Faculty of Veterinary Medicine Department of Surgery from November 2007 to February 2015 were reviewed for spinal tumors diagnosed by MRI and confirmed histologically. The results of neurological examination, neurological grade, conventional radiography findings, MRI findings, lesion localization, treatment modalities and outcomes were also evaluated [18].
Clinical examination: General physical, clinical and orthopedic examinations, and later neurologic examination scheme for spinal disease [27] were carried out, and paraplegia was graded according to the paraplegia score for dogs reported by Levine et al. [18]. In all dogs, complete blood count, serum biochemical profile, thoracic radiography and abdominal ultrasonography were performed to determine possible metastasis. Clinical outcomes were followed by neurological examination of the changes in neurological status and clinical findings after operation.
MRI acquisitions
All MRI examinations were performed with a 1.5 Tesla MR machine (Vision Plus; Siemens, Germany). T1 (time of repetition [TR]/time of echo [TE], 400/20 ms) and T2 (TR/TE, 2900/96 ms) weighted (W) turbo-spin echo images were obtained with 2 mm slice thicknesses. Gadolinium diethylenetriaminepentaacetic acid (Magnevist; Bayer, Germany) was used as the paramagnetic contrast medium, and was administered (dose, 0.2 mmol/kg) intravenously. All images were evaluated by board certified human neuroradiologists (OA) and veterinary surgeons (OB, PC).
Surgical technique
Laminectomy or hemilaminectomy were conducted to reach the lesion or remove the mass. The tumors were removed under the operation microscope in all cases. In two cases with osteosarcoma and one case with osteoma, spondylectomy was conducted to remove the mass. The created defects were filled with polymethylmethacrylate.
Histological examination
Histologically collected samples of each mass were fixed with 10% buffered formalin embedded in paraffin and cut to 5 µm-thick sections. All sections were stained with hematoxylin and eosin (H&E), phosphotungstic acid and Masson's trichrome stains. Some sections were stained using pan-cytokeratine, S-100, glial fibrillary acidic protein, α-smooth muscle actin (α-SMA) and vimentin (Dako, USA).
Outcome
The outcomes were provided after neurological examination and/or by telephone conversation with the owner or referring veterinarians.
Results
Eighteen dogs and one cat matched the case selection criteria for this study. The clinical, MRI, histological examination and surgical outcomes are summarized in Table 1. The sex distribution was 12 males and 6 females for dogs, as well as one female cat. Median age at presentation was 7.68 years (range, 2 to 12 years old), and median body weight for dogs was 16 kg (range, 7 to 52 kg). The breed distribution was mixed breed (n = 7), Sharpei (n = 1), Cocker Spaniel (n = 2), Poodle (n = 4), Siberian Husky (n = 1) long haired Collie (n = 1), Rottweiler (n = 1) Golden Retriever (n = 1), and one domestic short hair cat.
Table 1: Clinical, magnetic resonance imaging (MRI), histopathological examination and surgical outcomes in canine and feline patients with spinal tumors.
G0, no voluntary movement seen when the dog is supported; G1, intact limb protraction with no ground clearance; G2, intact limb protraction with inconsistent ground clearance; G3, intact protraction with ground clearance > 75% of steps; G4, ambulatory with consistent ground clearance and significant paresis-ataxia that results in occasional falling; G5, ambulatory with consistent ground clearance and mild paresis-ataxia that does not result in falling; G6, normal gait [18]. +, moderate enhancement; ++, good enhancement. MI, male intact; MN, male neutered; FI, female intact; FN, female neutered; yr, year; Lt, left; Rt, right; HL, hind limbs; DPP, deep pain perception; Hypo, hypointense; Hyper, hyper intense; CME, contrast-material enhancement.

Meningioma was seen in five dogs and one cat (66.66% of all intradural-extramedullar tumors). Localization of the tumor was in the lumbar area in three cases, in the cervical area in two cases, and in thoracic area in one cat. The clinical signs were progressive and in different degrees of neurological dysfunction. Characteristic MRI findings of all cases with meningioma were seen as hypointense on T1-weighted (T1W), slightly hyperintense on T2-weighted (T2W) MR sequences, and contrast-material enhancement on postcontrast T1W images. Meningeal tail was seen in two cases on postcontrast T1W images. Generally, the location of meningiomas was intadural-extramedullar on MRI. Cases were treated surgically, except when the owner opted for euthanasia. The longest survival time with better outcome was seen for sarcomatous meningioma (> 6 month). Neurological improvement was not observed in one case for one month after surgery, after which it was lost to follow up (case No. 3). The remaining two dogs survived for 3 and 5 months. The cat with meningioma underwent a second surgery because of tumor recurrence after 3 months, but neurological status was not improved after another one month, and it was then euthanized at the request of the owner. Histologically, the most common pattern was laminated whorls of elongated cells. Neoplastic cells had variably distinct borders with moderate cytoplasm, oval to fusiform shape and occasionally vesicular nuclei and nucleolus.
A dog with osteosarcoma in the T12 vertebra showed acute onset paraplegia with intact deep pain perception that disappeared 48 hours later. However another case with osteosarcoma in C2 had progressive ataxia and cervical pain. Characteristic MRI findings of osteosarcoma as sclerotic and lytic areas were seen. Both showed good contrast enhancement on postcontrast T1W images. Partial spondylectomy was conducted to remove the gross tumor completely in both cases, and the created cavity was filled with polymethylmethacrylate (PMMA) (T12) and screw + polymethylmethacrylate (C2). The case with cervical osteosarcoma was euthanized on day 46 and the case with thoracic osteosarcoma euthanized on day 21 because of pulmonary metastasis. Osteoma was diagnosed in a 2 year old dog with a history of progressive paraparesis. The radiopaque and lytic areas were prominent upon direct radiography. The tumor appeared in T7–T8 vertebral bodies, extended to the epidural space and pedicles and compressed the spinal cord from the right side. The tumor was hypointense on both T1W and T2W images, and showed ring like heterogenous contrast-material enhancement on postcontrast T1W images (Fig. 1). The tumor size was 29 × 33 mm on MRI, and it was completely removed by hemilaminectomy and partial spondylectomy, which created a defect that was filled with PMMA. Histologically, it was a well differentiated ovoid bone tumor (Fig. 2) in which the cells had hyperchromatic nuclei that were eccentrically located in dark stained cytoplasma and produced thin spicules of tumor osteoid and matrix.
Figure 1: Osteoma. Lateral X-ray image showing marked bony proliferation over the 8th–9th thoracic vertebrae (arrow in A). T2-weighted (T2W) images show hypointense mass (arrows in B and D). Coronal plane fat-suppressed postcontrast image demonstrates right paraspinal venous engorgement (broken arrows) and lytic-destructive lesion (arrow) with gadolinium enhancement (C). Contrast-material enhanced axial T1-weighted (T1W) images obtained at the level of T7–T8 show heterogeneous contrast-material enhancement and spinal canal narrowing (arrows in E and F).
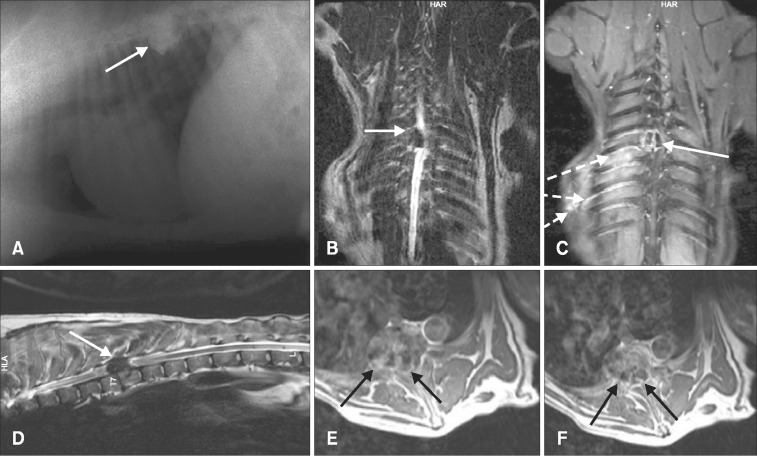
Figure 2: Well differentiated bone tissue (arrow; osteoma). H&E stain. 250×.

Clinical manifestation of cases with metastatic tumor consisted of neurologically weighted symptoms, which were the chief complaints proposed by the owner, and clinical signs were progressive. Metastatic spinal tumor was diagnosed in four cases and malign mesenchymal tumor, squamous cell carcinoma (panels A and B in Fig. 3), adenocarcinoma and rhabdomyosarcoma was diagnosed histologically. Tumors were multifocal, unshaped, and hypointense on T1W and hyperintense on T2W images. All tumors were located epidurally except for one case with metastatic mesenchymal tumor (case No. 10), in which it invaded the spinal cord.
Figure 3: (A) Contrast enhanced mid-sagittal T1 weighted image of thoracolumbar spine of a dog with metastatic squamous cell carcinoma. Metastatic tumors were contrast enhanced in T1W images (arrow heads). (B) Metastatic squamous cell carcinoma. H&E stain. 100× (B).

Nerve sheath tumor was diagnosed in four dogs, all of which had progressive proprioceptive ataxia, pain and different degrees of paresis, as well as atrophy of the related muscles. The tumor was located epidurally in all cases except for one with recurrence of clinical signs, and on the second MRI the mass was seen as both extradural and intradural (Case No. 15). Even though the mass was completely removed during the second operation, the neurological status was not improved and the dog was euthanized in response to the owner's request one month later. Hemilaminectomy with rhizotomy was conducted in all cases with nerve sheath tumor, and they survived for 2 or 3 months, although one case was lost to follow up after 1 month (Case No. 16). Nerve sheath tumor was diagnosed in one dog at L4–L5. After this tumor was removed surgically, clinical improvement was seen; however, the dog died from an unrelated disease 1 month later. Histologically, the tumor was well circumscribed without encapsulation and mainly composed of spindle-shaped cells with thin and eosinophilic cytoplasms and atypical plump, ovoid, and vesicular nuclei.
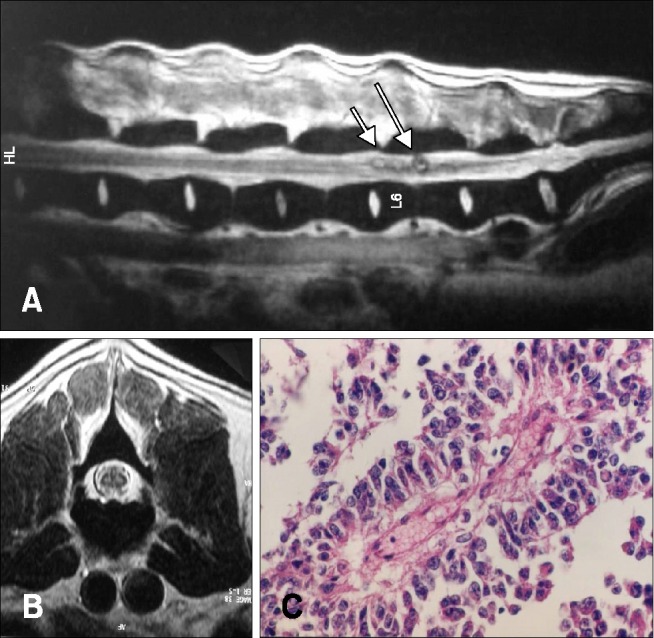
An intramedullary solid lesion that was confirmed histologically as ependymoma at L6 and accompanied diastematomyelia was removed surgically, but neurological status deteriorated and the animal was euthanized (panels A and B in Fig. 4). Upon histological examination, the ependymoma was composed of pseudorosettes, and immunohistochemical examination revealed vimentin and glial fibrillary acidic protein immunoreactivity in the neoplastic cells (panel C in Fig. 4).
Figure 4: Mid-sagittal T2W image of lumbar spine (A) transverse T2W image at the level of L6 (B) in a dog with ependymoma hyperintense masses located in the spinal cord parenchyma in T2W images. (C) Pseudorosette with central fibrovascular stroma (ependymoma). H&E stain. 400× (C).
One case with a mass located at the T12 level that was intradural–extramedullary and mimicked meningioma was histologically diagnosed as nephroblastoma. This dog was euthanized without any treatment. Histologically, the tumor was composed of uniform cells that were cuboidal to ovoid with indistinct cell borders and scant to mildly amphophilic. Immunohistochemically, neoplastic cells, especially in tubules and glomeruloid structures, stained pan cytokeratin. Conversely, fibrovascular stroma and capsules were stained α-SMA and vimentin sera.
Discussion
Characteristic signs and location of spinal tumor in MRI provided ante-mortem prediction of the various histological types of neoplasms. However, histological confirmation should be conducted for definitive diagnosis. Surgical removal of spinal tumors without neurological derioriation after the operation was observed for all except one case with ependymoma in this study, which indicates potential for treatment by gross total tumor removal and providing specimens for histological examination.
Older and larger breeds of dogs have been reported to be more susceptible to spinal tumors [5,15]; however, there may be a tendency for younger animals to develop tumors of neural origin [9,19,24]. The mean age of the dogs presented in this study was 7.68 years (range, 2–12 years), which is similar to that reported in the literature. In this case series, the youngest dog (2 years old) had nephroblastoma that occurred between 6 months and 3 years of age, but it has also been reported in dogs as old as 7 years [19,31].
Clinical signs related to spinal tumors can be noticed by the owner earlier before presentation to the veterinarian. This interval was reported to be about 1 month in a recent study [25]. The clinical signs of a spinal tumor represent spinal cord dysfunction and pain, but these are not pathognomonic [6,14,28]. In the present study, the time of determining abnormal clinical signs was not clear, and in some cases the owners could not remember the exact time of onset of neurogenic disorders. The clinical signs were progressive in all cases except for one case with osteosarcoma, in which acute onset of clinical signs was noticed by the owner. The later situation could be related to the growing mass invading to the epidural space and causing damage to the neural structure.
Distribution of spinal tumors according to location relative to the dura mater and histopathology has been reported as intramedullary (approximately 15%) [26], extradural (approximately 50%) and intradural-extramedullary (approximately 35%), which does not differ greatly from the observations in humans [23,24,26,31]. In the cases reported in this study, intramedullary tumor location was observed in one ependymoma and one metastasis case (2/19–10.5%), while the rest were intradural extramedullary 9/19 (47.37%) and extradurally 8/19 (42.10%). One case with a nerve sheath tumor was recurrent intradurally and extramedullary, even though it was located extradurally in the first MR study.
Dogs with meningiomas, which are the most common primary spinal tumors in dogs, usually admit with spinal pain, ataxia and paresis or paralysis according to neuroanatomic localization. Characteristic MRI findings are represented in humans and dogs as iso- to hypo-intense on T1W images and slightly hyperintense on T2W images. In addition, they generally show homogeneous enhancement with gadolinium, and dural tails are often present [6,22,23,30]. However, meningeal tail was not seen in four of the six cases in this study, indicating that this is not a pathognomonic MRI finding for meningioma. Meningioma revealed similar MRI findings in the present case series as observed in a previous study, and surgical outcomes regarding the early period were acceptable. Neurological improvement in meningioma cases had been reported for 17/24 (71%) of dogs [25]. The results of the case presented here were similar, with improvement seen in 4/5 (80%).
Different types of spinal nerve sheath tumors are recognized. Schwannomas, also known as neuromas or neurilemmomas, are usually solitary tumors, and are the most frequent type. If the nerve roots involved are not associated with the cervical or lumbar intumescence, the animal may only have spinal pain. The limb may be positioned in a more flexed posture (nerve root signature). Nerve sheath tumors showed different clinical signs according to anatomical location and usually progressed to proprioceptive impairment and sometimes to paraplegia or tetraplegia caused by spinal cord compression and related spinal cord pathophysiologic alterations. Nerve sheath tumors are isointense on T1W images and have an atypical marked high signal on T2w images [3,5,6,30]. In this case series, nerve sheath tumor was hypointense on T1W image and highly hyperintense on T2W images, and contrast-material was enhanced in all cases. Even though the tumor was located epidurally in the first operation, it had grown intradurally 2 months later and was completely removed after durotomy. Paraspinal muscle atrophy was seen in all cases, but it was more remarkable in two cases (case Nos. 14 and 15), and all cases with nerve sheath tumor showed progressive clinical signs.
Clinical signs of intramedullary ependymomas appeared in the later stage of the disease, and occurred in the conus medullaris and the filium terminale in humans [9,30]. Canine spinal cord nephroblastoma, which is also known as Wilms' tumor, generally occur between T10–L2 spinal cord segments in large breed dogs [19]. The ependymoma case presented in this study was admitted with hindlimb ataxia for 2 months. In addition to the tumor, there was diastematomyelia at the level of L6 in MRI, which deteriorated after surgical removal of the tumor. The case of nephroblastoma was accompanied by syringomyelia in MRI. Even though this case was not treated surgically, the owner allowed a biopsy, which enabled the case to be histologically confirmed.
Any malignant tumor can metastasize to bone, but the most common metastatic spinal tumors found in women are from the breast and lung, while those in men are from the prostate and lung in humans [30]. Metastases can also affect the spinal cord [8,21]. Both extradural and intramedullary metastasis is possible. Carcinomas are one of the most common types of tumors associated with extradural metastasis. In some instances, clinical signs of the metastasis may be apparent before clinical signs of the primary tumor [2]. In this case series, the first clinical signs noted by the owner were neurological signs that were located extradurally (n = 2), intramedullary (n = 1) and intradural extramedullary (n = 1), and none of them were treated surgically.
Bone tumors may be evident on survey radiographs as osteolytic/osteoproliferative processes. Classically, vertebral tumors do not cross the joint space (intervertebral disk); however, vertebral tumors do on occasion invade adjacent vertebral bodies and therefore appear to "jump" the joint. Extradural compression of the spinal cord overlying the vertebral body rather than the intervertebral disk space is indicative of neoplasia [2]. Spinal osteosarcoma was reported as 15% [15]. Both cases with osteosarcoma were predicted from survey radiography. However, the tumor margins and exact location with compressing spinal cord was diagnosed by MRI. Interestingly, in one case, acute onset paraplegia occurred. This case was treated with partial spondylectomy, and the created defect was filled with polymethylmethacrylate. Surgical procedures were well tolerated by the dogs, and their neurological status was not deteriorated during the early postoperative period. Partial spondylectomy for bone tumors and reconstruction with polymethylmethacrylate was found to be a versatile and cost effective method. However, wide surgical margins are essential for surgical oncology by radical spondylectomy, which involves more complex spinal instrumentation. Nevertheless, in both cases with osteosarcoma, the tumors were completely resected. This suggest that, if the radical spondylectomy and spinal instrumentation is combined with adjuvant or neoadjuvant therapy, the survival time could have been longer.
Osteoid osteoma and osteoblastoma are commonly seen as benign osteogenic bone neoplasms. Histologically, these tumors resemble each other, and if the lesion is larger than 1.5 cm it is accepted as osteoblastoma, which is most frequently located in the axial skeleton in humans. This tumor has a tendency to affect the posterior part of the vertebra and occurs primarily in the pedicle and the posterior elements, not in the vertebral body. As a result, this tumor often requires surgical resection. [1,30]. The case with osteoma presented in this study showed invasion of the tumor to the lateral part of the vertebra and that it included vertebral bodies larger than 1.5 cm. Even though the lesion was removed completely and the created defect repaired satisfactorily, radical spondylectomy and more sophisticated spinal instrumentation can be considered in humans to minimize recurrence. The case considered in this study was still alive with slight hindlimb ataxia at the time that this manuscript was written. To the best of the author's knowledge, this is the first case of spinal osteoma in dogs to be reported.
The method of choice for visualising spinal tumors is MRI. The tumor's involving structures, its relationship with the duramater, degree of spinal cord compression, and changes in spinal cord paranchyme can be visualised by MRI [11,29]. In this case series, all cases were predicted as tumors by MRI when compared with histological results. The characteristic MRI signs of the tumor were found to be reliable for diagnosis of spinal tumors and determination of the the surgical plane, and the multifocal appearance of the metastatic tumors on MRI differentiated them from other primary spinal cord tumors.
Extradural tumors are removed surgically, but both intradural/extramedullary and intramedullary tumors can be successfully resected [2]. Radiotherapy and chemotherapy are also treatment options that can be applied individually or in combination with surgery. In this case series, only surgical treatments were applied, and the results of surgery were found to be strategic except for intramedullary tumor (case No. 18), in which neurological status had deteriorated.
In conclusion, spinal neoplasia should be considered in cases with progressive neurological signs, and MRI is a useful and effective method for diagnosis of spinal tumors. Operative management is a strategic for epidural and intradural-extramedullary spinal tumors according to the surgical outcomes. Partial spondylectomy and reconstruction of vertebral bodies with polymethylmethacrylate can be suggested for bone tumors. Further studies are needed to correlate clinical findings, and characteristic signs, as well as the shape of tumor in MRI, histological findings and outcomes after different treatment.
Footnotes
Conflict of Interest: There is no conflict of interest.
References
Share this article / Teilen Sie diesen Artikel
Diese Webseite wurde mit Jimdo erstellt! Jetzt kostenlos registrieren auf https://de.jimdo.com








