
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Evaluations of phylogenetic proximity in a group of 67 dogs with
osteosarcoma: a pilot study
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Canine Periosteal Osteosarcoma
Images added / Abbildungen hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
New subcategory added / Neue Unterkategorie hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
Images added / Abbildungen hinzugefügt 01.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Heart valvular diseases



Hyperthermic Isolated Limb Perfusion with TNF α and Cisplatin in the Treatment of Osteosarcoma of the Extremities: A Feasibility Study in Healthy Dogs

Van Ginkel, Robert J. et al. “Hyperthermic Isolated Limb Perfusion with TNF Α and Cisplatin in the Treatment of Osteosarcoma of the Extremities: A Feasibility Study in Healthy Dogs.” Sarcoma 3.2 (1999): 89–94. PMC. Web. 21 May 2017.

Abstract
Purpose. The feasibility of hyperthermic isolated limb perfusion (HILP) with tumor necrosis factor-α (TNFα ) and cisplatin for the management of osteosarcoma was studied in the canine model.
Methods. During seven perfusions in six healthy mongrel dogs (weight 32±2 kg) technical aspects of HILP under mild hyperthermia (39– 40℃) were studied. In five experiments HILP was performed with TNFα alone (0.5 mg/l extremity volume), and in two experiments TNFα was combined with cisplatin (25 mg/l extremity volume). During the perfusions physiological parameters were monitored and TNFα and total cisplatin concentrations were determined.
Results. Perfusion conditions (pH, PCO2 , PO2, flow and pressure) remained within physiological ranges.Three dogs died within 24 h despite a sublethal systemic concentration of TNFα that leaked from the perfusion circuit. Three dogs were terminated; one dog after the second experiment in accordance with Dutch ethical rules; one dog showed an invagination of the small bowel resulting in an ileus; one dog because of necrosis of the perfused limb.
Conclusions. This feasibility study in healthy dogs demonstrated that HILP with TNFα and cisplatin was associated with a high mortality rate and does not allow us to treat dogs with spontaneous osteosarcoma with TNFα and cisplatin HILP. Therefore, an alternative model should be used in the search for the ideal combination of perfusion agents for limb sparing treatment in human osteosarcoma.
Introduction
Osteosarcoma is the most frequent primary malignant bone tumor in humans. Until the 1970s the most
common approach to the management of localized osteosarcoma was surgical resection, amputation or
radiation therapy.1 During the last decades a definite role for neoadjuvant high-dose methotrexate and
cisplatin-based polychemotherapy was established.1-4 The potential local tumor effect of systemically
administered cisplatin, however, is limited due to the nephrotoxicity and ototoxicity of cisplatin. Therefore
an attempt was made to increase the local effe ct of cisplatin without increasing systemic toxicity by using
hyper thermic isolated regional limb perfusion (HILP) with cisplatin in dogs with spontaneous osteosar-coma.5
These studies showed an acceptable locore-gional toxicity, improved functional outcome at 6
and 12 weeks, and a steadily improving radiological picture. However, the histological results were modest,
with none of the dogs showing a complete response at 6 weeks after perfusion. The same experience was
found in patients with sarcomas of soft tissue and bone treated with cisplatin HILP.6
Results of recent publications and of our own experience with a new perfusion modality, which combines tumor necrosis factor-a(TNFa) and melphalan in patients with recurrent melanoma or
soft tissue sarcoma, are very promising. 7,8 However, in six of eight evaluable patients with unresectable osteosarcoma of the lower limb treated wi th TNFaand melphal an HILP,
histological evaluation revealed moderate results with > 80% necrosis in three patients, 50-60% necrosis in two patients and < 50%
necrosis in one patient. After TNFaand melphalan HILP, limb spar ing surgery was possible in six patients.9 As cisplatin is one of the most active chemotherapeutics in the treatment of
osteosarcoma, it seems worthwhile to investigate the high frequency high frequency of occurrence in dogs, canine osteosarcoma is a useful model for evaluation of new treatment regimens in
humans as rapid case accrual and rapid time to reach measurabl e end poi nts are possible.10 The canine osteosarcoma therefore appears
to be a valid model for studying the potential treat-ment of HILP with TNFaand cisplatin in the local treatment of osteosarcoma of the extremity in humans. To establish optimal HILP
conditions using TNFaand cisplatin for local tumor control in dogs bearing osteosarcoma, a feasibility study in healthy dogs was undertaken.
Materials and methods
Dogs
During seven experiments in six healthy mongrel dogs with a mean average weight of 32 ± 2 kg and a mean
age of 6 ± 1 years, different aspects of HILP with TNFaand cisplatin were studied. Preoperatively, all
dogs were thoroughly clinically evaluated at the Central Animal Facility of the University of Groningen.
The study was approved by the Animal Welfare Committee of the Faculty of Medicine of the Gron-ingen
University.
Anaesthetics
The dogs fastened for 12 h and were anaesthetized with thiopental (30 mg/kg body wt., i.v.) (Pentothal,
Abbott, Amstelveen, The Netherlands) and, after musclerelaxation with pancuroni umbromide
(0.08 mg/kg body wt., i.v.) (Pavulon, Organon, Oss, The Netherlands), the dogs were ventilated (Ohmeda
Modulus 2) with a mixture of O2 and isoflurane. The oxygen concentration in the gas mixture was continuously
measured by means of an oxygen analyzer (Ohmeda Modulus 2) and minute volumes (4±6 l/ min) were adjusted to maintain an end-expiratory CO 2 concentration of 4-5% (Siemens CO 2 analyzer
930). The dogs were placed in the supine position on a heated mattress to maintain their normal body temperature of 38°C.11 During the
operations all
dogs were given about 2 l of glucose 5% via acephalicor internal jugular vein. Central arterial pressure was
recorded as well as an ECG and diuresis.
Operation and perfusion techniques
During anaesthesia the volume of the extremity was measured using Archimedes’ rule (1.7±2 l). The iliac vessels were exposed under sterile conditions and collateral vessels were clipped.
Cannulas were inserted into the artery (Bardic, 14-18 F) and vein (Bardic, 14-18 F). Both cannulas were connected to an extracorporeal circuit consisting of an occlusive roller pump, a
cardiotomy reservoir and a bubble oxygenator with heat-exchanger. A tourniquet made of nylon was placed around the base of the extremity, using a pin in the bone and a bandage around the
middle to complete the isolation of the limb from the systemic circulation. The perfusate consisted of 350 ml 5% dextran 40 in glucose 5% (Isodex, Phar macia AB, Uppsala, Sweden), 250 ml
red blood cells (canine blood donor s), 250 ml plasma, 30 ml sodium bicarbonate 8.4% and 0.5 ml 5000 IU/ml heparin ( Thromboliquine, Organon B. V. , Oss, The Netherlands). The mixture of
oxygen, air and carbon dioxide through the oxygenator was adjusted to maintain the blood gas values within the physiological range and, when necessar y, bicarbonate was added to adjust
the pH value. All perfusions were performed under mild hyperthermic conditions (39-40°C) and optimal physiological conditions.12,13
Thermistor probes ( Electrolaboriet , Copenhagen, Denmark) were inserted into the subcutaneous tissues and into a muscle of the thigh just above the knee for continuous monitoring of the
temperatures during perfusion. In the first five experiments TNFawas the sole perfusion agent, i n the l ast t wo experi ments TNFawas
combi ned with cispl atin. The dosage of TNFa (0.5 mg/l extremity volume) (Boehringer, Ingelheim,
Germany) was calculated in order not to exceed ten times the acceptable systemic levels (systemic , 10 mg/kg body wt).14 The dosage of cisplatin (20 mg/l extremity volume) (Platinol 0.5
mg/ml, Bristol Myers Squibb, Weesp, The Netherl ands) used in the perfusion had been established in a previous study and was based on a maximum tolerable dose of 30 mg/l extremity
volume.15 Cisplatin was added to the circulated perfusate in 10 min. During perfusion, serum TNFaand tot al ci spl ati n l evels were determined in the regional and systemic
circulation
at 0, 5, 15, 30, 45, 60, 75 and 90 min by ELISA and flameless atomic absorption spectophotometry (FAAS), respectively. The perfusion time was 1 h, followed by wash-out of the extremity
with 3 l of Isodex. Tourniquet, cannulas and clips were then removed and the incisions in the vessels repaired. Protamine hydrochloride (Hoffman La Roche,
Mijdrecht, The Netherlands) was administered, to neutralize heparin, in a ratio of 1:1 to the initial dose
of hepar in. All dogs were closely observed for at least 24 h. No anti-inflammatory or analgesic drugs were
administered during follow-up.
All dogs were followed for local and systemic side effects of TNFa and cisplatin perfusion, as well as survival.
Results
Table 1 shows the characteristics of the seven experiments in six dogs. During the experiments conditions for perfusions (pH, PCO 2 , PO 2 ) were kept within the physiological ranges, as in human perfusions. Figure 1 shows the flow, blood pressure, perfusion pressures, weight gain or loss of the extracorporeal circuit and temperature during 60 min of perfusion in the seven experiments.

In the first five experiments, only TNFa was administered to the perfusion circuit. In the last two experiments cisplatin was added. Figure 2 illustrates the TNFaconcentrations (mean ± SEM) in
the perfused limb as well as in the systemic circulation of the dog during perfusion and afterwards. Peak TNFa concentrations in the perfused limb were
650 ± 158 ng/ml, and in the systemic circulation of the dog they were 37 ± 15 ng/ml. The peak systemic
concentrations in the dog were in the same range as those of TNFa and melphalan HILP used in the
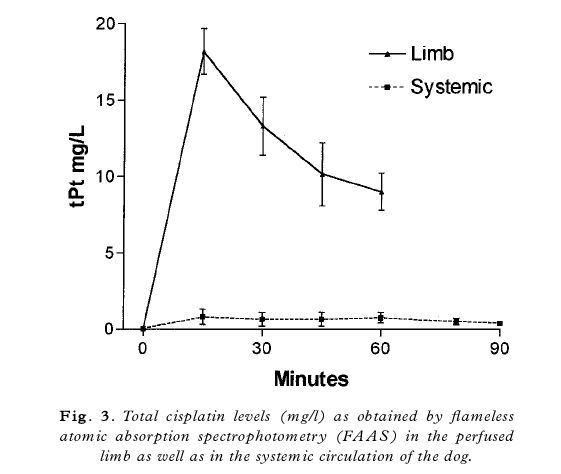
treatment of humans at our institute.16 Figure 3 shows the measured total cisplatin values in the last two
experiments. During the experiments we were not able to perform any leakage monitoring by means of radionuclear detection techniques which are used in the clinical perfusion setting. Therefore
leakage was calculated afterwards according to Stehlin with the amount of blood in the dogs estimated at 69 ml/kg body weight. 17 Calculated leakage values are summarized in Table 1.
Three dogs died within 24 h: the first two during the TNFa experiment; the third after TNFa and cisplatin perfusion. Postmortem examination of these animals did not provide any macroscopic or
microscopic evidence to explain the cause of death. Three dogs were terminated; two due to treatment complications. One dog showed an invagination of the small bowel, resulting in an ileus and
another was terminated 1 week after TNFaand cisplatin perfusion because of necrosis of the perfused limb. The third dog was terminated after the second experiment in accordance with Dutch ethical
rules.
Discussion
In the treatment of osteogenic sarcoma a distinction can be made between systemic therapy and locore-gi
onal treatment. High-dose methotrexate-based systemic chemotherapy is primarily administered in order to eradicate possible micrometastatic disease, and its use was a major breakthrough in the
clinical treatment of osteosarcomas in the 1970s.1,2 Today, about 60% of patients with resectable primary tumors and no metastases at the time of the initial diagnosis will be cured.1 The primary
objective in locoregional treatment is to prevent local recurrence and allow limb salvage procedures in an attempt to preserve limb function. New surgical techniques and the development of
endoprosthetic materials, coupled with systemic neoadjuvant chemotherapy, have offered less radical surgery for 40±80% of patients with osteosarcoma since the 1980s.1,18 Procedures that increase
tumor
necrosis of the primary tumor, with reduction of viable tumor cells and tumor volume, could contribute to limb preservation strategies. Since its ®rst use, cisplatin has been one of the most
effective chemo-therapeutic agents and has been incorporated in most systemic treatment regimens for osteosarcoma. A recent attempt to overcome its nephrotoxic and ototoxic limitations by
administering cisplatin in HILP in the treatment of spontaneous canine osteosarcoma was histologically modest.5 Promising results of recent publications and our own experience with a
new combination perfusion modality (TNFaand melphalan) for recurrent melanoma or soft tissue sarcoma, but moderate histological results in patients with osteosarcoma, prompted us to investigate
the combination of TNFaand cisplatin in HILP for osteosarcoma.7-9 Since endothelial cells are supposed to play a key role in the working mechanism of TNF, osteosarcomas with a high extent of
tumor vessels are of particular interest. Before application of TNFaand cisplatin HILP in humans and client-owned osteosarcoma-bearing dogs, the present feasibility study was performed in normal
healthy dogs. Despite sufficient experience in HILP in dogs as well as in humans, an unexpected high mortality rate was encountered. Although there was no mortality related to the operation,
three dogs died
within 24 h after perfusion (50%). This direct postoperative mortality could not be explained by a surplus of systemical leakage of TNF. In the experi-ment, the dog with the highest leakage and,
as a consequence, the highest systemic TNFaconcentra-tions, survived immediately postoperatively, and the dog with the lowest leakage (lowest systemic TNFa concentrations) died within 24 h after
perfusion. No correlation between leakage and mortality rate could be established. Maximal leakage encountered in these experiments was 33%, this corresponds to 330 mg
TNFagiven systemically per dog; since the average dog weighs 33 kg, the dose of TNFathat reaches the systemic circulation of the dog is sublethal (10 mg/kg).14


Although only sublethal doses of TNFaleaked to the systemical circulation, the clinical picture resembled responses obser ved with lethal doses ( >100 mg/ kg) , character i z ed by progressive
hypotension, shock and death within 24 h.
Due to the lack of facilities, we were not able to support the dogs with intensive postoperative care, as is the case
after human TNFaHILP. In par t this could explain the obser ved direct postoperative mor tal ity and supports the need for intensive treatment after TNFa HILP in the dog. Three dogs survived the
®rst days after perfusion;
however, one dog developed an i l eus and was terminated within 1 week after perfusion. One dog that underwent two experiments survived the first without morbid effects, but was terminated after
the second experiment according to Dutch ethical rules. Leg toxicity consisted in slight erythema and edema in all dogs except one in the cisplatin-treated group. In this dog, necrosis of the
perfused l imb was encountered, necessitating termination. We have never observed necrosis of the perfused limb with the cisplatin dose used (25 mg/l extremity volume) in
experiments where cisplatin was the sole perfusion agent.15 This observation may indicate that TNFa might enhance the effect of cisplatin to the local tissues of the perfused limb. The in vitro
anticancer potential, and overcoming cisplatin resistance with the combination of TNFaand cisplatin in different cell lines, has been established by others.
20-22 Buell et al. demonstrated an increased cellular cisplatin accumulation and DNA adduct formation as the
possible cellular basis for the augmented cisplatin cytotoxicity in the presence of TNF and hyper ther-mia.23
Recently, Anda et al. demonstrated that TNFa selectively promoted the in vitro permeability of the blood.brain barrier to CDDP without disrupting the tight junctions.24

An improved penetration of cisplatin into the interstitial space due to a higher permeability of the vascular wall, combined with an increased cellular cisplatin accumulation and DNA adduct for mation, could explain the observed necrosis of the limb in this in vivo model with the cisplatin dose
used, which was previously non-toxic. The observed mor tality and morbidity that we encountered in this canine study was similar to the experience of Withrow and colleagues (unpublished
observations). The present results in normal elderly mongrel dogs indicate that treatment of dogs with spontaneous osteosarcoma using TNFaand cisplatin
HILP is not appropriate. Future research could focus on postoperative monitoring and care in dogs after
TNFaHILP; perhaps a better alternative for testing the effect of TNFawith cisplatin HILP is the use of the rat osteosarcoma model described by Manusama et al.,25 since rats are much less
susceptible to TNFa than dogs.
Acknowledgments
The advice and technical assistance, in the Groningen experimental TNFaand cisplatin perfusion
study for spontaneous canine osteosarcoma, of A.P. Gaaikema, W.A. Buurman PhD, W.M. Molenaar MD
PhD, D.R.A. Uges PhD, E.G.E. de Vries MD PhD, J. de Vries MD PhD and M.M. Weggemand-Reuvers
are very much appreciated. This study was supported by a grant from the Groningen Surgical Department.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ham SJ, Schraffordt Koops H, van der Graaf WT, van Horn JR, Postma L, Hoekstra HJ. Historical, current and future aspects of osteosarcoma treatment. Eur J Surg Oncol. 1998 Dec;24(6):584–600. [PubMed]
- Rosen G, Tan C, Sanmaneechai A, Beattie EJ, Jr, Marcove R, Murphy ML. The rationale for multiple drug chemotherapy in the treatment of osteogenic sarcoma. Cancer. 1975 Mar;35(3 Suppl):936–945. [PubMed]
- Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick JA, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986 Jun 19;314(25):1600–1606. [PubMed]
- Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987 Jan;5(1):21–26. [PubMed]
- Van Ginkel RJ, Hoekstra HJ, Meutstege FJ, Oosterhuis JW, Uges DR, Schraffordt Koops H. Hyperthermic isolated regional perfusion with cisplatin in the local treatment of spontaneous canine osteosarcoma: assessment of short-term effects. J Surg Oncol. 1995 Jul;59(3):169–176. [PubMed]
- van Ginkel RJ, Schraffordt Koops H, de Vries EG, Molenaar WM, Uges DR, Hoekstra HJ. Hyperthermic isolated limb perfusion with cisplatin in four patients with sarcomas of soft tissue and bone. Eur J Surg Oncol. 1996 Oct;22(5):528–531. [PubMed]
- Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992 Jan;10(1):52–60. [PubMed]
- Eggermont AM, Schraffordt Koops H, Liénard D, Kroon BB, van Geel AN, Hoekstra HJ, Lejeune FJ. Isolated limb perfusion with high-dose tumor necrosis factor-alpha in combination with interferon-gamma and melphalan for nonresectable extremity soft tissue sarcomas: a multicenter trial. J Clin Oncol. 1996 Oct;14(10):2653–2665. [PubMed]
- Withrow SJ, Powers BE, Straw RC, Wilkins RM. Comparative aspects of osteosarcoma. Dog versus man. Clin Orthop Relat Res. 1991 Sep;(270):159–168. [PubMed]
- Thrall DE, Page RL, Dewhirst MW, Meyer RE, Hoopes PJ, Kornegay JN. Temperature measurements in normal and tumor tissue of dogs undergoing whole body hyperthermia. Cancer Res. 1986 Dec;46(12 Pt 1):6229–6235. [PubMed]
- Fontijne WP, Mook PH, Elstrodt JM, Schraffordt Koops H, Oldhoff J, Wildevuur CR. Isolated hindlimb perfusion in dogs: the effect of perfusion pressures on the oxygen supply (ptO2 histogram) to the skeletal muscle. Surgery. 1985 Mar;97(3):278–284. [PubMed]
- Fontijne WP, de Vries J, Mook PH, Elstrodt JM, Oosterhuis JW, Schraffordt Koops H, Oldhoff J, Wildevuur CR. Improved tissue perfusion during pressure-regulated hyperthermic regional isolated perfusion in dogs. J Surg Oncol. 1984 May;26(1):69–76. [PubMed]
- Tracey KJ, Lowry SF, Fahey TJ, 3rd, Albert JD, Fong Y, Hesse D, Beutler B, Manogue KR, Calvano S, Wei H, et al. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987 May;164(5):415–422. [PubMed]
- Zwaveling JH, Maring JK, Clarke FL, van Ginkel RJ, Limburg PC, Hoekstra HJ, Koops HS, Girbes AR. High plasma tumor necrosis factor (TNF)-alpha concentrations and a sepsis-like syndrome in patients undergoing hyperthermic isolated limb perfusion with recombinant TNF-alpha, interferon-gamma, and melphalan. Crit Care Med. 1996 May;24(5):765–770. [PubMed]
- STEHLIN JS, Jr, CLARK RL, Jr, WHITE EC, HEALEY JE, Jr, DEWEY WC, BEERSTECHER S. The leakage factor in regional perfusion with chemotherapeutic agents. Arch Surg. 1960 Jun;80:934–945. [PubMed]
- Meyer WH, Malawer MM. Osteosarcoma. Clinical features and evolving surgical and chemotherapeutic strategies. Pediatr Clin North Am. 1991 Apr;38(2):317–348. [PubMed]
- Eichenholz PW, Eichacker PQ, Hoffman WD, Banks SM, Parrillo JE, Danner RL, Natanson C. Tumor necrosis factor challenges in canines: patterns of cardiovascular dysfunction. Am J Physiol. 1992 Sep;263(3 Pt 2):H668–H675. [PubMed]
- Mutch DG, Powell CB, Kao MS, Collins JL. In vitro analysis of the anticancer potential of tumor necrosis factor in combination with cisplatin. Gynecol Oncol. 1989 Sep;34(3):328–333. [PubMed]
- Mizutani Y, Bonavida B. Overcoming cis-diamminedichloroplatinum (II) resistance of human ovarian tumor cells by combination treatment with cis-diamminedichloroplatinum (II) and tumor necrosis factor-alpha. Cancer. 1993 Aug 1;72(3):809–818. [PubMed]
- Sleijfer S, Le TK, de Jong S, Timmer-Bosscha H, Withoff S, Mulder NH. Combined cytotoxic effects of tumor necrosis factor-alpha with various cytotoxic agents in tumor cell lines that are drug resistant due to mutated p53. J Immunother. 1999 Jan;22(1):48–53. [PubMed]
- Buell JF, Reed E, Lee KB, Parker RJ, Venzon DJ, Amikura K, Arnold S, Fraker DL, Alexander HR. Synergistic effect and possible mechanisms of tumor necrosis factor and cisplatin cytotoxicity under moderate hyperthermia against gastric cancer cells. Ann Surg Oncol. 1997 Mar;4(2):141–148. [PubMed]
- Anda T, Yamashita H, Khalid H, Tsutsumi K, Fujita H, Tokunaga Y, Shibata S. Effect of tumor necrosis factor-alpha on the permeability of bovine brain microvessel endothelial cell monolayers. Neurol Res. 1997 Aug;19(4):369–376. [PubMed]
- Manusama ER, Stavast J, Durante NM, Marquet RL, Eggermont AM. Isolated limb perfusion with TNF alpha and melphalan in a rat osteosarcoma model: a new anti-tumour approach. Eur J Surg Oncol. 1996 Apr;22(2):152–157. [PubMed]
- Wieberdink J, Benckhuysen C, Braat RP, van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982 Oct;18(10):905–910. [PubMed]
Share this article / Teilen Sie diesen Artikel
Diese Webseite wurde mit Jimdo erstellt! Jetzt kostenlos registrieren auf https://de.jimdo.com








